A randomized study carried out at Tata Memorial Centre showed a disease-free and overall survival benefit for pre-operative progesterone in patients with node positive breast cancer, independent of their progesterone receptor (PR) status [J Clin Oncol, 2011. 29(21): p. 2845-51]. However, the underlying biological mechanism by which progesterone exerts the aforementioned effect remains unclear.
We describe proteome profiling of PR-positive and PR-negative breast cancer cells using a phospho-kinase array in response to progesterone treatment. Our analysis suggests that progesterone induces dephosphorylation of kinases (specifcally EGFR, AKT and ERK1/2) that are directly involved in regulation of invasion and migration of breast cancer cells. Consistent with our biochemical results, we show that progesterone inhibits breast cancer cell invasion and migration independent of the PR-status of the cells [1].
Next, we performed deep sequencing for small RNA in four breast cancer cell lines treated with progesterone. Our analysis led to a surprising discovery of a novel feedback mechanism regulating the expression of PR. We show that miR-129-2 gets up-regulated in response to progesterone treatment that in turn targets the 3’UTR of progesterone receptor PR. We further demosntrate that targetting miR-129-2 using anti-miR restores the expression of PR in breast cancer cells. Consistent with this finding, TCGA analysis of primary breast cancer tumors suggests an increased expression of miR-129-2 in PR-negative patients compared to PR-positive ones [2].
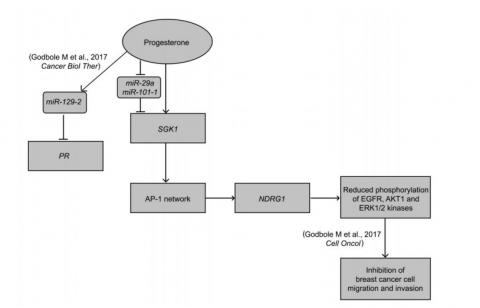
More recently, to understand the molecular basis of how progesterone affects the outcome of PR-negative cells we further performed an integrated analysis of micro array based mRNA expression profile and deep sequencing of non-coding small RNA of breast cancer cells. We present an intricate convergence model indicating a dual-phase regulation downstream to progesterone treatment to regulate the expression of a Serum- and Glucocorticoid-regulated Kinase gene 1, SGK1: predominantly driven as a direct transcriptional target, in PR-positive breast cancer cells; and, down-regulation of miR-29a and miR-101-1 targeting SGK1 with relatively distinct effect in PR-negative breast cells in response to progesterone. We demonstrate with a series of over expression and shRNA knockdown assays, along with biochemical validations, that the stringent up-regulation of SGK1 in response to progesterone lead to the activation of a tumor metastasis suppressor gene, NDRG1, via a set of AP-1 network genes that inactivates AKT1, ERK1/2 and EGFR kinases, impeding the invasion and migration of breast cancer cells [3].
In summary, we propose a model for the mode of action of progesterone in breast cancer deciphering the molecular basis of a randomized clinical trial studying the effect of progesterone in breast cancer. Such preoperative endocrine therapies, in contrast to neoadjuvant chemotherapy, are much simpler and economical to deliver. An understanding of the targets, as outlined in this study, could thus be of immense potential utility in monitoring the response of hormones in human cancer.
The figure summarizes our study where progesterone treatment of breast cancer cells increases the expression of SGK1, which up -regulates NDRG1 via the AP -l network genes, independent of the PR status of the cells. We also show that progesterone suppresses the expression of miR -29a and miR - 101 - 1 targeting 3’UTR of SGK1, a dual -regulatory mode of expression of SGK1 in breast cancer. The increased expression of NDRG1 causes reduction in phosphorylation of kinases and thus suppresses cell invasion and cell migration of the cells. The model also summarizes our previous results where we have shown that progesterone -mediated up -regulation of miR-129-2 decreases the expression of PR in breast cancer. Thus the model provides a molecular basis to the clinical findings of preoperative progesterone intervention in breast cancer.

Assistant Professor
Dr. Mukul Sacchit Godbole
Institute of Bioinformatics and Biotechnology, Pune