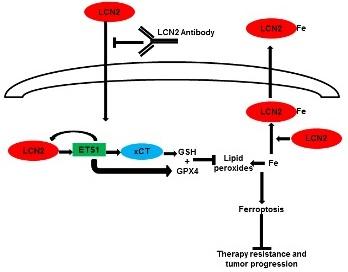
Our previous work demonstrated that loss of the desmosomal plaque protein, plakophilin3 (PKP3), leads to an increase in tumor progression and metastasis in multiple cell types. We have since identified multiple events downstream of PKP3 loss that are essential for tumor progression including the stabilization of the intermediate filament protein keratin8 (K8) and an increase in the expression of MMP7 and LCN2. Our results also indicate that LCN2 was required for the increase in invasion and tumor progression observed upon PKP3 loss. Further work from the laboratory has since demonstrated that PKP3 loss leads to radio-resistance and resistance to 5 flourouracil (5FU) in colon cancer cell lines. The resistance is dependent on LCN2 expression in multiple colon cancer cell lines and is independent of PKP3 levels in these cell lines. LCN2 mediated resistance is due to the ability of LCN2 to inhibit a novel cell death process, ferroptosis. Ferroptosis is an iron dependent cell death mechanism that is distinct from other forms of cell death such as apoptosis and necroptosis. The induction of ferroptosis is dependent on the presence of reactive iron radicals and an inability to reduce peroxidated phospholipids either due to a decrease in the levels of the enzyme glutathione peroxidase4 (GPX4), or due to a decrease in glutathione (GSH) production due to a decrease in Cystine import by system xc-. Our data suggest that LCN2 inhibits ferroptosis in two ways: by inhibiting the accumulation of ferrous iron in cells and by stimulating the expression of GPX4 and xCT, a component of system xc-. Therefore, expression of LCN2 inhibits ferroptosis by inhibiting the accumulation of reactive iron species and by reducing peroxidated lipids (figure 1). Further, inhibiting LCN2 expression with a monoclonal antibody reverses chemo and radio resistance and inhibits tumor progression in xenograft assays suggesting that it could serve as the basis for a potential therapeutic. This is important as LCN2 is over-expressed in many tumor types including colorectal cancer and is an indicator of colon cancer progression from adenoma to carcinoma. LCN2 over-expression leads to increased tumor formation in xenograft models of colon cancer and therefore LCN2 can serve as both a marker of resistance and a potential therapeutic target in multiple tumor types. In addition, we have generated compound transgenic mice that express the APCmin allele and have specific loss of PKP3 in the intestine. These mice show increased disease progression and develop a rectal prolapse. IHC studies indicate that the mice show increased LCN2 and xCT levels. This combined with our data from colon tumor samples validates the results we have observed in colon cancer cell lines and suggest that this model might be an excellent system in which to test single chain and humanized variants of the LCN2 antibody.