
ERBB2 and KRAS Alterations Mediate Response to EGFR Inhibitors in Early Stage Gallbladder Cancer
Clinical collaborator:Dr. Shailesh Shrikhande (TMC)
The uncommonness of gallbladder cancer in the developed world has contributed to the generally poor understanding of the disease and thus lends itself to the need for further research. While the 5-year survival rate of an early stage T1 gallbladder carcinoma is nearly 100%, it signficantly decreases as the disease progresses with less than 15% for T3/T4 advanced stage tumors. A hope for longer term survival has specifically been promising for an early staged T2 carcinomas with an intermediate 5-year suvival. The development of new and effective treatment has been and thus continues to be a major public health imperative. We perform an integrated analysis of a phospho-proteomic array profile of tyrosine kinase; whole exome sequencing; copy number analysis; and, histo-chemical analysis in a rare set of 44 predominantly early-stage gallbladder tumor samples, followed by a thorough cell-based functional assay, and biochemical analysis using in vitro and in vivo systems. Our integrated analysis indicates ERBB2 alterations in 40% early-stage rare gallbladder tumors through overexpression in 24% (n=25) and recurrent mutations in 14% tumors (n=44); along with co-occurring KRAS mutation in 7% tumors (n=44). We demonstrate that ERBB2 heterodimerize with EGFR to constitutively activate the ErbB signaling pathway in gallbladder cells. Consistent with this, treatment with ERBB2-specific, EGFR-specific shRNA or with a covalent EGFR family inhibitor Afatninb inhibits tumor-associated characteristics of the gallbladder cancer cells. Furthermore, we observe an in vivo reduction in tumor size of gallbladder xenografts in response to Afatinib is paralleled by a reduction in the amounts of phospho-ERK, in tumors harboring KRAS (G13D) mutation but not in KRAS (G12V) mutation, supporting an essential role of the ErbB pathway. In overall, this study establishes KRAS mutant allele as the potential predictive marker for anti-EGFR drug response in gallbladder cancer, wherein presence of KRAS (G12V), but not KRAS (G13D) mutation, may preclude gallbladder cancer patients to respond to anti-EGFR treatment
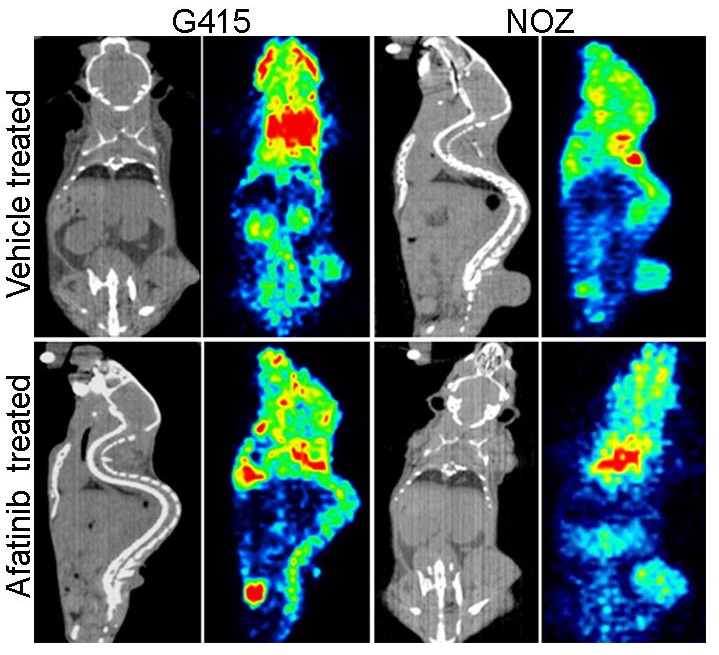
Figure: In vivo sensitivity of gallbladder cancer cell lines to EGFR inhibitor: CT scan and PET imaging by F18-FDG uptake is shown for vehicle (upper panel) and afatinib (lower panel) treated xenografts of G415 and NOZ gallbladder cells with endogenous activated ErbB signaling. Xenograft of both G415 and NOZ cells forms tumors in mice. G415 cells that co-harbors a weaker activating allele of KRAS (G13D) responds to EGFR inhibitor treatment, as seen with significant reduction in tumor size, while the NOZ cells harboring KRAS (G12V) mutation are not responsive. This data suggests that gallbladder cancer patients harboring KRAS (G12V) mutation along wth ERBB2 alteration are likely to be refractory to EGFR inhibitor therapy.